During atrophy, desmin intermediate filaments depolymerization precedes and accelerates myofibril destruction.
The predominant cytoskeletal network in muscle, desmin intermediate filaments, is critical for the mechanical and structural integrity of the myofibrils. Desmin filaments are localized between adjacent myofibrils, linking them laterally to the Z-lines, and between myofibrils and the sarcolemma, mitochondria, and nuclear membrane. Desmin filaments aligned alone the Z-lines, where actin thin filaments are anchored and contribute to their stability. We discovered that during atrophy, desmin filaments are phosphorylated, disassembled, and degraded, and their loss precedes and accelerates myofibril destruction.
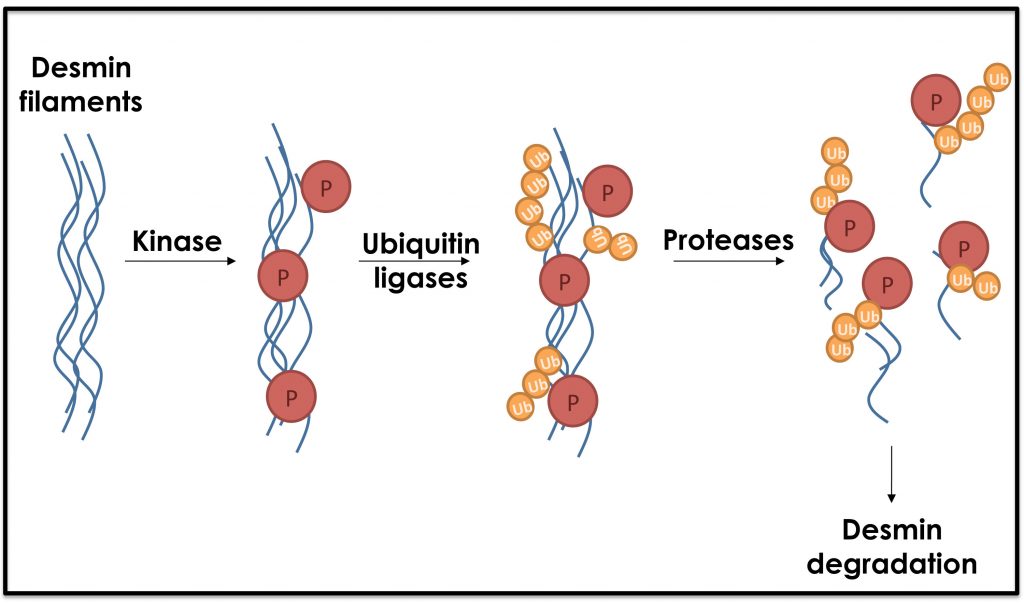
We focus on the distinct individual proteolytic steps that lead to myofibril loss during rapid or slow atrophy, and specifically on the structural changes of filamentous assemblies (i.e. desmin), whose loss accelerates myofibril destruction. We use electron microscopy, stochastic optical reconstruction microscopy, and biochemical approaches to acquire a nano-resolution of these critical structures, and to characterize the subcellular organization and structural properties of the desmin cytoskeleton in normal and atrophying muscle. The combination of these powerful techniques together with the development of in vitro assembly regimes will increase our understanding of the molecular mechanisms leading to desmin disassembly during atrophy, and the effect of their depolymerization on myofibril loss and muscle architecture. Our goal is to identify critical key players in this debilitating process of atrophy in order to facilitate the development of new rational therapies.